|
|
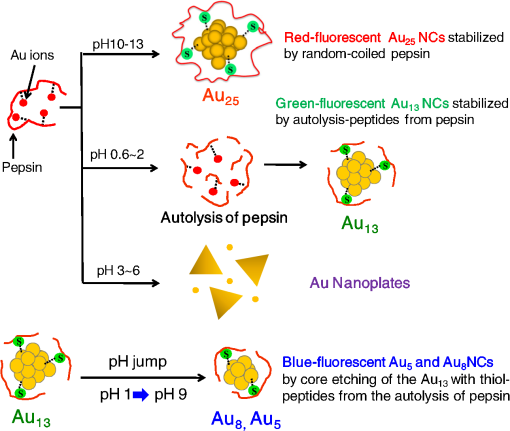
1.IntroductionGold nanoclusters are attracting a wealth of attention in many areas of nanotechnology. These sub-nanometer particles demonstrate molecular-like electronic transitions between HOMO-LUMO energy levels, due to their finite cluster size.1,2 As a result, energy transitions can be rationalized according to the jellium model ().2,3 Due to this unique electronic nature of gold nanoclusters, photoluminescent properties are prominent in these nanomaterials,2,4–9 generating new opportunities for optical applications. Gold nanoparticle (Au NP) synthesis is a well-explored field in nanotechnology,10–12 reaching across many areas such as chemistry, physics, and biology. However, gold nanocluster (Au NC) synthesis is a relatively new field still in development. The isolation and purification of Au NCs has been a recent scientific achievement, leading to many thorough investigations of both its structure and its implications for nanotechnology.13–15 The crystal structures of and 16,17 NCs have motivated researchers to analyze their novel structures with a variety of experimental methods. Au NCs are already being recognized as potential catalytic agents.18–20 Computational and theoretical studies have also played a big part in probing the electronic structure.1,14,21 Some of the unique electronic effects of Au NCs can be attributed to a surface staple-like bonding structure between the organic capping ligand and surface gold atoms (see Fig. 1).22–24 This is just one feature rendering Au NCs to be an exciting nanomaterial for future research. Fig. 1A model of the with surface staple motif “S-Au-S-Au-S”. Organic capping ligands are omitted for clarity of the structure. 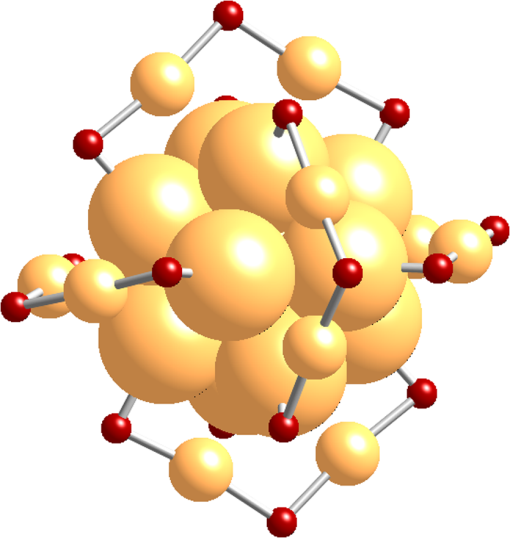 The effect of the capping/stabilizing ligand has been demonstrated to have a profound effect on the photoluminescence of Au NCs,6,25,26 which in turn, influences possible optical applications. An interesting example of modifying the capping material is utilizing proteins and other biomolecules to reduce and arrange gold atoms into stable Au NCs. Many proteins contain active sites for metal ion accumulation and reduction where Au NCs can form and be stabilized. Depending on the protein and reaction conditions, Au NCs can be formed with decent fluorescence intensity. It is not until recent years that we have witnessed a dramatic increase in publications investigating fluorescent protein-Au NCs.27 Researchers are continually exploring interactions inside these hybrid systems of proteins and metallic particles, establishing new ground for nanomaterials.28,29 The intense photoluminescence observed from these protein-stabilized Au NCs has been reported in a number of studies27 and is promising for replacing less biocompatible quantum dots used for imaging and targeting applications.30 New studies strive to improve and optimize the photoluminescence property for applications such as toxic molecule detection31 and biolabeling.32 Only a few studies thus far have reported some intriguing insights into the structure of protein-Au NCs and how they are formed under the influence of the protein33–35. This review will begin with the synthesis of protein-stabilized Au NCs and then move into the recent investigations of their structure. The final section will explore viable fluorescence applications that have been reported in the literature and the outlook for protein-Au NCs. 2.Synthetic MethodsThiol-capped Au NPs have been extensively studied in many fields of nanotechnology because of strong Au—S interactions on the NP surface, leading to highly stable Au NPs.12,36–38 Proteins, such as lysozyme and bovine serum albumin, containing sulfur-bearing amino acids, have been functionalized as nanoparticle thiol-capping agents a few years prior to the intense investigation of nanoclusters.39–41. This demonstration of protein-assembled nanostructures, along with many earlier contributions,28,29 excited the field of nanotechnology with the new idea of forming nanoparticles in protein templates. However, understanding how these highly ordered structures synthesize nanoparticles is demanding, requiring an extensive knowledge of protein-nanoparticle interactions.42,43 Some recent contributions will be discussed. Biomineralization is a natural process in which biological organisms intake metal species to subsequently form mineral structures. Stimulated by this process, research has shown that nanostructures can be formed when harnessing the biological organism’s or macromolecule’s ability to naturally intake and arrange inorganic materials.44–47 From this previous fundamental work, incorporating proteins with gold atom precursors has evolved into a new branch of one-pot syntheses, producing stable and fluorescent Au NCs. This pathway for synthesizing nanoparticles and nanoclusters has been referred to as “protein/peptide-directed,” “biomineralization,” and “protein-stabilized,” to name a few. There are many advantages to this one-pot synthetic approach, including its relatively low environmental impact, which is very attractive in today’s environmentally conscience world.48 Mild reaction conditions, aqueous solution chemistry, and absence of strong reducing agents make the formation of Au NCs a green chemical synthesis. Quite often, only pH conditions in the reaction are altered to optimize the protein’s reducing ability or to change the conformation to increase organic-metal bonding and stabilization. A typical reaction pathway for a protein-directed synthesis is shown in Fig. 2. Fig. 2This reaction scheme illustrates the facile synthetic route for producing Au NCs with selected proteins that promote NC growth and formation.  Since the protein plays a vital role of stabilizing, reducing, and arranging gold atoms into stable nanoclusters, the formation of a single Au NC product is highly dependent on the conformation of the protein.33 A highlight of these water-soluble Au NCs is how readily they can be used in applications because of their immediate biocompatibility. Already, many proteins have been used as stabilizing agents for Au NCs such as BSA,49–54 lysozyme,55,56 human transferrin,57 lactoferrin,58 tryspin,59 pepsin,60 insulin,61 and horseradish peroxidase.62 It is already known that select amino acids can play a key role in nanoparticle synthesis due to their reducing strength and/or favorable binding interactions with gold.63,64 When amino acids are randomly organized in peptide strands, only certain sequences of amino acids can promote nanoparticle growth while others will have little interaction with metal ions.65,66 Working toward this end, a “bottom-up” study performed by Lee et al.67 tested all 20 amino acids to determine their reducing and binding capabilities when interacting with . After documenting the ability of each individual amino acid, combinations of active amino acids were synthesized into peptide chains to capture the effect of neighboring amino acid residues in a secondary conformation. Tryptophan was identified for being the strongest reducing agent and was therefore interdigitated into custom peptides for its reducing property. In a similar fashion, histidine was selected as the strongest metal binding amino acid and was also interdigitated into another set of peptides. From these preliminary peptide results, more diverse combinations with other amino acids were investigated to determine the resultant shape and size of the Au NPs, along with tracking the periods of reaction initiation, growth, and termination. The increase in complexity from small peptides to larger protein systems is extremely significant. In a few studies of protein-stabilized Au NCs and NPs, structural changes of proteins have been detected using techniques such as UV-Vis, circular dichroism, infrared and mass spectrometry.33,35,43 Pal et al.33 proposed an interesting scheme for how the growth of protein-Au NPs could be an autocatalytic process, where the protein initially reduces gold atoms to form a Au NP seed, which then promotes further intake into the protein. This study used human serum albumin, subtilisin Carlsberg, and an E. coli extract to obtain kinetic parameters for Au NP formation. From this kinetic data and other proteins tested, they also proposed that the induction period length for initializing Au NP growth could be influenced by the protein’s melting temperature, a useful insight for optimizing the formation of Au NPs and Au NCs. 3.Structural Investigations3.1.Standard Characterization MethodsA wealth of techniques have been developed and applied for understanding Au NPs over the past few decades. From these well-established methods, only some have proved useful for investigations of protein-Au NCs. For instance, because of magnification limitations for transmission electron microscopy (TEM), Au NCs are not as easily imaged as Au NPs. High-resolution TEM can be attempted to detect Au NC size at the subnanometer level but is not always successful. In particular, Au NC species smaller than 25 gold atoms have been difficult to observe.60 Conventional spectroscopies, such as UV-Vis and fluorescence, are essential in protein-Au NC studies to capture their optical properties. However, protein-Au NCs are too small to exhibit surface plasmon resonance (SPR); therefore, no absorption features in the visible region clearly detect the presence of Au NCs. Fluorescence spectroscopy is, of course, imperative for reporting excitation and emission wavelengths, as well as fluorescence lifetime events. Infrared and circular dichroism spectroscopy can be useful for identifying changes to the protein structure before and after synthesis, confirming any protein denaturation. In addition to these standard characterization techniques, more robust methods are providing valuable information on the structural environment of protein-Au NCs. 3.2.Structural Determination by Mass SpectrometryAn important characterization tool for the elucidation of thiol-capped Au NCs is mass spectrometry.15,37,68 Mass spectrometry alone can provide reliable structural details, such as determining the size of the gold core and capping ligand environment. Obtaining these parameters can lead to a quantitative analysis of Au NC composition. Determining the precise sizes of the () magic cluster series has initiated major breakthroughs in quantum calculations of nanoclusters, leading to thorough explorations of their electronic structure.1,14 Numerous articles continue to be published, revealing interesting properties and potential applications for the cluster. After the introduction of the first protein-Au NCs, mass spectrometry was proven to be an important tool for identifying cluster sizes inside the capping protein. It has also been shown to be a good technique for tracking the formation of Au NCs.69 There has been some speculation surrounding the actual size of the protein-Au NCs to determine if they are indeed magic clusters (i.e., or ). So far, protein-Au NCs have been reported as both single magic clusters49,52,60 and a distribution of cluster sizes.54,57,58 This discrepancy could stem from the difference in the protecting protein. Nevertheless, further research is needed to make such conclusions. One study35 utilized matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) to document the pH-dependent and time-dependent in situ growth of Au NCs in BSA and native lactoferrin protein. From this insightful study, MALDI-MS results highlighted the importance of pH and reaction time on the protein’s reducing and stabilizing ability. With neutral pH levels, was found to bind with the protein as a complex with only 13 to 14 gold atoms found in the cluster. At an alkaline pH level (), which is commonly used in protein-directed syntheses,49,55 further reduction to was observed leading to larger clusters forming around 25 gold atoms. Some interesting observations were the presence of free, unreacted proteins as well as ions that were redistributed at different times in the reaction. Without the addition of NaOH to the lactoferrin protein and ion mixture, only 17 gold atoms were found in the product. Adding NaOH to the mixture produced a 25 gold atom product after 8 h. After 24 h the same reaction mixture had amounts of unreacted protein along with the 25 gold atom Au NC, indicating that redistribution of ions is evident in the protein-directed process. 3.3.Structural Determination by X-ray TechniquesOn the topic of probing the structure of protein-Au NCs, there are x-ray spectroscopy techniques that offer a site-specific determination of local structure for Au NCs. A few x-ray spectroscopies are mentioned below to discuss how they are indispensible for uncovering protein-stabilized Au NC structure and understanding the protein-directed process. One technique commonly seen in Au NC and NP research is x-ray photoelectron spectroscopy (XPS). Examining the and binding energies of protein-stabilized Au NCs is an excellent method for determining the size and overall oxidation state of gold by comparing spectra to -thiol ligand and gold foil. Peak fitting of XPS spectra can determine a quantitative measurement for composition. Standard in a number of Au NC studies is the and peaks will often lie between and , indicating a compositional mixture of a core with surface atoms. Many of the red-emitting Au NCs found in the literature have anywhere from 10% to 25% of .54,57,61 The binding energy of the 4f peaks will also shift to a higher energy as the Au NC size shrinks.70 Contrary to the NC, only composition is detected for blue-emitting clusters with no gold-thiol bonding indicated at the S 2p peaks.54 It was hypothesized that the cluster is loosely held inside the protein. A comparison is shown in Fig. 3. Fig. 3XPS spectra of the Au 4f band for and . A shift in higher binding energy is seen for smaller clusters. From peak fitting of each band, mostly is only seen for where has some contribution, likely from the NC surface. (See Ref. 55). 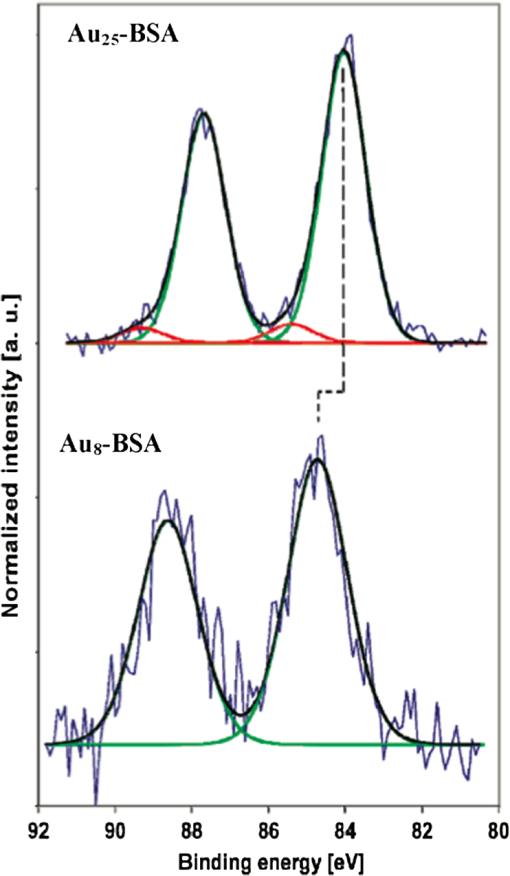 Y. Lu et al. undertook a remarkable study34 gaining a mechanistic point of view of how a protein and metal precursors interact to produce gold nanoparticles. Recent convention for synthesizing protein-Au NCs has been to start with salt and protein in solution.49,55 However, this study used a single native lysozyme crystal with incorporated salt to observe the time-dependent arrangement of gold inside the protein crystal by achieving slower kinetic formation of nanoparticles. With x-ray crystallography and electron microscopy, the distribution and arrangement of gold atoms inside the lysozyme were tracked over several days, observing a disproportionation of to and , where accumulates to yield larger Au NPs up to 20 nm (Fig. 4). The amino acid residue histidine is an accumulation source for before it becomes disproportionated. This observation deems histidine a vital component for the protein-directed growth of Au NCs and NPs. This study created a new vision for observing bio-nano hybrid systems and identifying important functional residues of the protein crucial for gold nanoparticle and nanocluster growth. Fig. 4A schematic representation of disproportionating to form Au NPs in a lysozyme crystal. (See Ref. 34).  X-ray absorption spectroscopy (XAS) can determine structural parameters of a chemical system from an element-specific perspective. It can be broken down into two parts: x-ray absorption near-edge spectroscopy (XANES) and x-ray absorption of fine structure (XAFS). In a typical experiment, a polychromatic light source (obtained from synchrotron radiation) is tuned to excite the core electrons of a certain element (i.e., gold), which then emits a photoelectron wave interacting with the surrounding atoms in its bonding environment. The resulting re-absorption from the returning photoelectron wave contains information about the number of surrounding atoms, their element type, and bond lengths. Zhang et al.22 used these techniques, along with complementary calculations to compare the electronic structure between the BSA-stabilized Au NC and the known cluster. From structural refinement results and comparison to previous results, it was determined that the BSA-stabilized cluster may also have the unique surface staple structure as seen in the cluster. Also from this study, densities of states (DOS) calculations were done to illustrate the electronic influence from the staple structure compared to that of the nanocluster core. The difference between the surface staple and the core has a dramatic effect on the entire cluster, which helps explain the interesting properties Au NCs behold. Additional research remains to confirm the existence of the surface-staple in fluorescent protein-Au NCs. A recent study synthesized paired Au NCs inside a ferritin protein enhancing and red-shifting the fluorescence compared to a single unpaired Au NC.71 This group examined XANES spectra at the Au -edge of Au-amino acid mixtures, Au NPs and their synthesized paired ferritin-Au NCs. Comparing the edge-shift from XANES and binding energy from XPS, they were able to determine the composition as well as confirm the important role of histidine in the reduction and formation of ferritin-Au NCs. 4.Optical Properties and Potential Applications4.1.Gold Nanocluster Optical PropertiesProtein-stabilized Au NCs are being recognized for their strong fluorescence in the visible spectrum. The discovery of Au NC photoluminescence was reported earlier, stating that luminescence will intensify with decreasing nanoparticle size.4 This transition can be seen where SPR from Au NPs diminishes and photoluminescence occurs at the Au NC size regime. It is known that the photoluminescence property is attributed to molecular-like transitions between HOMO-LUMO energies1–3 due to their ultra-small size. In result, the interband radiative transition lifetime is very short for Au NCs, ranging from picoseconds to nanoseconds.72 Dickson et al. has provided many investigations of dendrimer-stabilized Au NCs exhibiting a wide fluorescence range.3 Dickson’s work has demonstrated that Au NCs closely follow the jellium model when determining transition energies. They have also shown the size-dependent effect on surface potentials as the potential wells change from a spherical harmionic (Au NCs) to a square well (Au NPs).2 In these experiments it was observed that quantum yields were as high as 70% for to 10% for , all having fluorescence lifetimes of a few nanoseconds. However, these results are from dendrimer-encapsulated Au NCs. Varying the capping molecule can dramatically affect the fluorescence properties of the Au NC system. To explore the effects of the protecting molecule on fluorescence properties, investigations are under way. Sakanaga et al.73 investigated excited energy emission bands of the nanocluster and isolated two luminescence transitions, one band between 6sp states and the excited band between 6sp and 5d states. Work from Jin et al.25 prepared the cluster capped with various ligands and demonstrated the trend of increasing fluorescence intensity by ligand’s ability to donate electron density to the Au NC surface. The total oxidation state of was shown to influence the luminescence intensity as it increases with a more positive oxidation level on the cluster. Based on ongoing research, more can be achieved in order to maximize quantum yields and understand the origin of fluorescence. 4.2.Sensing ApplicationsThe detection of toxic metals (, , ) in solution is a possible analytical application for Au NPs.74–77 It is shown that interactions with heavy metal ions can be detected by changes in the UV-Vis absorption due to the aggregation or chelation of Au NPs.78,79 Therefore, Au NPs can serve as colorimetric assays for toxic metal detection. More recently, Au NCs capped with proteins and other biomolecules have also been employed for selective detection of metals as an application. Experimenting with BSA has been a perfect starting point for researchers to study protein-Au NC systems. Xie et al.49 ignited this interest for using BSA when they introduced a facile protein-directed synthesis, which was subsequently adopted by many research groups for other protein-Au NC systems. BSA has been used previously42,43 as a capping agent for Au NPs but not in a biocompatible fashion. As a result of this accessible synthesis, a surge of BSA-Au NC studies emerged.50–54 Red emission from BSA-Au NCs occurs around 640 nm with quantum yields reported as high as 6% (see Fig. 5).49,51 The BSA-Au NC was found in a few reports50,54,80 to have a selective interaction with species. This interaction quenches the fluorescence linearly with increasing concentration. It has also been shown that BSA-Au NCs can detect other metals such as .52 Fig. 5Comparison between BSA (blue) and red-emitting BSA-Au NC (red), both in solution and in lyophilized form. The absorption spectra are the dashed lines and fluorescence spectra are the solid lines. Inset image is the excitation spectrum of BSA-Au NC. See Ref. 48). 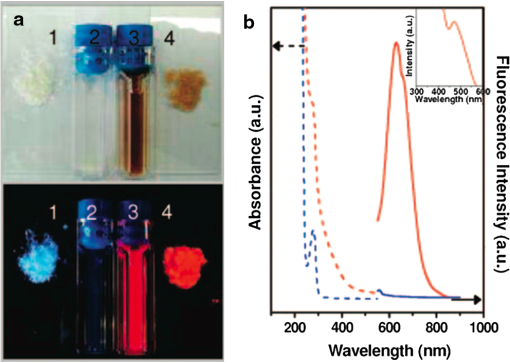 There have been a few proposed mechanisms for fluorescence quenching discussed in the literature. One suggested mechanism is that highly metallophilic bonding between the centers, and , disrupts the fluorescence from Au-BSA interactions.80 The second proposed mechanism is a photo-induced electron transfer process. In this process, Hg-S bonds are formed from BSA, which then intercepts one of the charge carriers during the excitation process, reducing to . It is thought that the latter species creates interference, thereby quenching the fluorescence.50 Cyanide etching is one known process that will quench BSA-Au NC fluorescence by removing gold atoms from the gold core.81 Most detection limits for sensing applications have been very low, ranging from 80 nM50 to 0.5 nM,80 ultrasensitive for determining toxic metal concentrations in an environmental setting.81 Another study53 has employed BSA-Au NCs for the biodetection of glutaraldehyde with a detection limit of 0.2 µM. This fluorescence-quenching event is caused by crosslinking between BSA and glutaraldehyde. Another protein of choice for experimentation with bio-nano systems is lysozyme because of its commercial availability and antibacterial properties. Lysozyme has been confirmed to synthesize Au NCs in a protein-directed process with near IR fluorescence. Like BSA, it is no surprise that lysozyme would be a good candidate for nanocluster growth as it has already been employed for synthesizing Au NPs.39,82,83 One of the first lysozyme-stabilized Au NC was synthesized by Lu et al.,55 reporting an emission wavelength of 657 nm and a quantum yield of 5.6%. The application of detection was also proven to be viable with a detection limit of 10 nM, comparable to Xie et al.80 with 0.5 nM. Indeed, lysozyme-Au NCs have the detection ability despite difference in protecting protein. Taking the lysozyme-Au NC one step further, one group was able to synthesize similar clusters to the previous study on an eggshell membrane, which contains the lysozyme protein high in cysteine units.56 Again, a green protein-directed approach is used. This study’s results produced Au NCs on a solid-state platform with near IR emission similar to Lu et al.55 This group proposed applications for this fluorescent nanomaterial such as recyclable catalysts and chemical sensing paper. Since the initial breakthrough of using BSA and lysozyme, other biomolecules of various sizes have successfully synthesized fluorescent Au NCs, which also exhibit detection capabilities. Au NCs stabilized with the amino acid l-cysteine72 showed fluorescence emission in the blue end of the visible spectrum () and was used as a detection probe for glucose sensing. This study also tested for glucose levels in serum samples with selective detection avoiding interference from other serum proteins. Glutathione was used as a stabilizing biomolecule,84 producing similar red emission to BSA and lysozyme Au NCs but with an emission wavelength further red-shifted, correlating with the larger size of the observed nanocluster (). Glutathione-stabilized Au NCs showed fluorescence quenching from ions and good stability against photobleaching and oxidation, making them potential bio-imaging probes. The iron-binding protein lactoferrin stabilized red-emitting Au NCs while also being useful for detecting ions.58 Fluorescence quenching was minimal with the addition of iron ions, indicating the lactoferrin protein structure was relatively undisturbed in the final product. The lactoferrin-Au NC also exhibited good stability at various pH levels, maintaining strong fluorescence. This study investigated the specific region where the Au NC formed in the lactoferrin by using Förster resonance energy transfer (FRET). This technique detects energy transfers from excited donors to acceptors, which can reveal the energy transfer efficiency and the nature of the bonding between lactoferrin and the Au NC. Another protein, trypsin, can be used as a stabilizing agent for Au NCs59 with red emission at 640 nm, selective detection, and resistance to photobleaching. The stability against photobleaching was comparable with CdSe quantum dots, making them a relatively nontoxic alternative for bio-imaging. Besides quenching the fluorescence in order to detect other species, protein-Au NCs have been able to detect other metal species with metal-enhanced fluorescence or luminescence (MEF or MEL).52,60,80 This event occurs when metal NPs are close in space but not directly bound to the fluorophore. Enhanced fluorescence from metal species is due to a number of effects, such as altering the radiative decay rate and increasing the absorption of the fluorescing molecule.85 In the case of protein-Au NCs, the protein acts as a boundary layer between the NPs or other metals and Au NC to enhance the fluorescence. Examples of MEF or MEL with protein-Au NCs are the detection of Ag NPs, and 52,60,80 4.3.Tunable EmissionIn the preceding section, most of the protein-stabilized Au NCs discussed have red to near IR fluorescence originating from Au NCs with an average size of 25 gold atoms. It was found that if Au NCs are synthesized to extremely small clusters (sometimes called nanodots) with dendrimers or an etching process5,86,87 they will exhibit intense blue fluorescence. Here arises an interesting structure-property relationship between Au NC size and their emission wavelength where the fluorescence blue shifts with a decreasing number of gold atoms in the cluster. Protein-mediated synthesis proves to be quite flexible, especially when subtle changes to the reaction can yield modified Au NCs with different emission properties. An example of this is a study54 where NCs were synthesized with BSA by lowering the pH to 8. This is a slight change in pH from the original pH of 11 to 12 for red-emitting NCs in BSA. The emission wavelength for NCs was 450 nm, comparable to the previously synthesized blue NCs but with a quantum yield that is slightly lower, around 6%. Other small Au NCs were synthesized in another study88 with an emission wavelength of 490 nm (bluish-green) and a declared size for the cluster of . These NCs were not encapsulated by a large biomolecule but stabilized by histidine. All of these blue-emitting Au NCs have shown high quantum yields and could be accessible for bio-imaging purposes. Similar to the pH-modified synthesis of BSA-Au NCs to yield , a recent study demonstrated how the gastric peptide pepsin could be adaptable to produce blue, green, and red fluorescent Au NCs,60 schematic shown in Fig. 6. In alkaline pH conditions, red-emitting NCs were stabilized by pepsin, similar to BSA and lysozyme. Strong acidic conditions produced NCs stabilized by autolytic peptide strands from pepsin with a subsequent green fluorescence. To reduce the core size to blue-emitting and , a dramatic jump to higher pH effectively etched the core to this final size. XPS was used to compare the 4f peaks for , , and , which showed increasing binding energy with reduced core size. The effect of Au NC size on the fluorescence emission energy is presented well in this study. This protein-directed route for pepsin was successful for utilizing various conformations and, in this case, cleaved residues to synthesize an array of Au NCs. The NC was shown to be useful for the detection of and by fluorescence quenching and fluorescence enhancement, respectively. 4.4.Biolabelling and Targeted Imaging ApplicationsReturning to the BSA-Au NC, highlighted in this review, a couple of studies51,52 released shortly after Xie et al.49 were able to conjugate folic acid to the surface of BSA through amine linkages. These studies used Au NCs as an imaging probe for detection of folate receptors on () oral carcinoma cells with an emission profile extending into the near-IR, suitable for tissue penetration. Results reveal these Au NCs as nontoxic and a viable method for selectively detecting and imaging cancer cells since only a minor reduction in the quantum yield was detected after the bioconjugation of folic acid. From this study, it could open possibilities to conjugate linkages in the BSA structure for other receptor-targeting biomolecules for targeted imaging applications. The protein-directed synthesis was adapted to produce human apo-transferrin-stabilized Au NCs with strong emission in the near IR region for cell imaging and targeting.57 As described previously, reducing agents are avoided for protein-directed syntheses to improve biocompatibility and retain native protein structure. In this study, however, the mild reducing agent ascorbic acid was added to aid the reduction of gold ions without adding excessive amounts of protein. The iron-free apo-transferrin protein initially stabilized the Au NC with iron added afterwards to determine if apo-transferrin maintains its natural iron-binding ability. The addition of iron was found to have little effect on the fluorescence of the Au NC, indicating preservation of the transferrin protein. A cell study demonstrated transferrin-Au NCs can enter lung tumor cells and maintain strong red fluorescence. High stability against photobleaching, changes in solution pH, and different buffer systems were also reported. From this study alone, a new protein-stabilized Au NC was synthesized with immediate applications for targeting and imaging cancer cells. On the topic of preserving protein functionality, insulin has been successfully utilized as a stabilizing agent for red-emitting Au NCs.61 However, insulin lacks cysteine residues, therefore very few Au-S bonds are present in the structure so other amino acids must exercise their binding ability to stabilize the Au NC. The size from HRTEM images indicated a particle size of , where MS data was unable to confirm the composition. Interestingly, we still see a comparable fluorescence emission profile to other protein-Au NCs and a large amount of with small surface composition of , determined from XPS. Insulin-Au NCs showed great biocompatibility and cell internalization (see Fig. 7). During cell studies, it was found that insulin-Au NCs could also be used as a contrasting agent for computed tomography along with fluorescence imaging, making these Au NCs a dual functioning imaging probe. As for the bioactivity, insulin-Au NCs were comparable to commercially available insulin in lowering the blood glucose levels in small animal tests. Many impressive features of the insulin-Au NC have been shown and hold great potential for future biomedical technology. Fig. 7Internalization of fluorescent insulin-Au NCs inside myoblast cells. The as-prepared Au NCs retain their strong red fluorescence inside the cell. Fluorescent staining of the cell nucleus (blue) and cell wall (green) is also shown. (See Ref. 60). 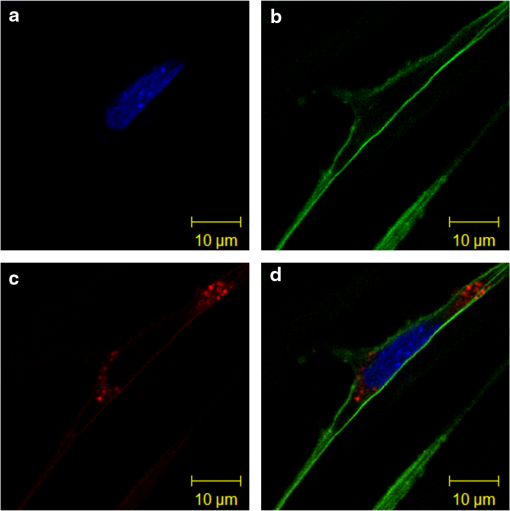 The enzymatic protein, horseradish peroxidase, successfully stabilized fluorescent Au NCs.62 The horseradish peroxidase enzyme proved to be a good stabilizing agent for the growth of Au NCs with appreciable red fluorescence. It was observed that the enzyme retains its enzymatic ability to breakdown , leading to quenching of the Au NC fluorescence. TEM and XPS results indicated there was Au NC aggregation leading to larger Au NPs, consequently quenching the fluorescence. These examples of protein-Au NCs serving as biolabeling and imaging probes demonstrate the multifunctional abilities capable for immediate biomedical applications. Advantages for using protein-Au NCs over other fluorescent nanomaterials are their water solubility, low toxicity, and facile synthesis, excluding harsh reducing agents or organic solvents. Protein-Au NC systems are also multifunctional, enabling further modifications of its structure by conjugating additional biomolecules to its surface. For example, work done with red light-emitting dihydrolipoic acid capped Au NCs demonstrated the versatile nature of these systems.89 Instead of directly stabilizing the Au NC with proteins (mainly discussed in this review), a bioconjugation step attached functional biomolecules such as BSA, PEG, avidin, and streptavidin. This study presented Au NCs enduring a number of synthetic steps while preserving strong luminescence and stability while also exploiting the bioconjugated Au NCs as a biolabeling agent. Additional studies exploring the functionality of the capping environment are anticipated, especially for targeted imaging and drug delivery applications. 5.Conclusions and OutlookThe study of protein-Au NCs is evidently a quickly evolving field in nanotechnology. Several proteins and other biomolecules are successful stabilizing agents for Au NCs yielding highly stable products with promising light-emitting applications. Protein-Au NCs presented in this review have shown very low detection limits for fluorescence quenching detection, good photostability for biolabeling/imaging purposes and in some cases, preservation of native protein structure to retain biological activity. With green chemical syntheses of these nanomaterials, researchers will able to conduct in-depth studies investigating biomedical applications without further biocompatibility preparations. Time- and pH-dependent studies illustrated in this review have identified important factors for understanding the protein-directed synthesis of Au NCs. As a result of these new bio-nanomaterials, knowledge of protein-Au interactions has greatly improved from the recent progress made in this field. However, only a few studies thus far have contributed investigations of Au NC local structure although many studies have been able to report various cluster sizes with mass spectrometry. Further investigation of the protein-nanocluster bonding environment remains to be explored. Even though protein-Au NCs show initial signs of good biocompatibility, it is expected that many more in vivo and in vitro experiments will be conducted before further integration into biomedical applications beyond the current research level. With protein-Au NCs still in their infancy, many rising applications, such as sensitive fluorescence detection and biolabeling, appear to be possible in the upcoming years. AcknowledgmentsThe authors would like to thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for financial support in the form of Discovery Grants. ReferencesM. Zhuet al.,
“Correlating the crystal structure of a thiol-protected cluster and optical properties,”
J. Am. Chem. Soc., 130
(18), 5883
–5885
(2008). http://dx.doi.org/10.1021/ja801173r JACSAT 0002-7863 Google Scholar
J. ZhengP. R. NicovichR. M. Dickson,
“Highly fluorescent noble metal quantum dots,”
Ann. Rev. Phys. Chem., 58
(1), 409
–431
(2007). http://dx.doi.org/10.1146/annurev.physchem.58.032806.104546 ARPLAP 0066-426X Google Scholar
J. ZhengC. ZhangR. M. Dickson,
“Highly fluorescent, water-soluble, size-tunable gold quantum dots,”
Phys. Rev. Lett., 93
(7), 077402
(2004). http://dx.doi.org/10.1103/PhysRevLett.93.077402 PRLTAO 0031-9007 Google Scholar
J. P. Wilcoxonet al.,
“Photoluminescence from nanosize gold clusters,”
J. Chem. Phys., 108
(21), 9137
–9143
(1998). http://dx.doi.org/10.1063/1.476360 JCPSA6 0021-9606 Google Scholar
J. ZhengJ. T. PettyR. M. Dickson,
“High quantum yield blue emission from water-soluble nanodots,”
J. Am. Chem. Soc., 125
(26), 7780
–7781
(2003). http://dx.doi.org/10.1021/ja035473v JACSAT 0002-7863 Google Scholar
G. Wanget al.,
“Near-IR luminescence of monolayer-protected metal clusters,”
J. Am. Chem. Soc., 127
(3), 812
–813
(2005). http://dx.doi.org/10.1021/ja0452471 JACSAT 0002-7863 Google Scholar
T. P. BigioniR. L. WhettenÖ. Dag,
“Near-infrared luminescence from small gold nanocrystals,”
J. Phys. Chem. B, 104
(30), 6983
–6986
(2000). http://dx.doi.org/10.1021/jp993867w JPCBFK 1520-6106 Google Scholar
Y. Baoet al.,
“Nanoparticle-free synthesis of fluorescent gold nanoclusters at physiological temperature,”
J. Phys. Chem. C, 111
(33), 12194
–12198
(2007). http://dx.doi.org/10.1021/jp071727d 1932-7447 Google Scholar
D. Leeet al.,
“Electrochemistry and optical absorbance and luminescence of molecule-like nanoparticles,”
J. Am. Chem. Soc., 126
(19), 6193
–6199
(2004). http://dx.doi.org/10.1021/ja049605b JACSAT 0002-7863 Google Scholar
J. ShanH. Tenhu,
“Recent advances in polymer protected gold nanoparticles: synthesis, properties and applications,”
Chem. Commun.,
(44), 4580
–4598
(2007). http://dx.doi.org/10.1039/B707740H Google Scholar
M. Grzelczaket al.,
“Shape control in gold nanoparticle synthesis,”
Chem. Soc. Rev., 37
(9), 1783
–1791
(2008). http://dx.doi.org/10.1039/b711490g CSRVBR 0306-0012 Google Scholar
R. Sardaret al.,
“Gold nanoparticles: past, present, and future,”
Langmuir, 25
(24), 13840
–13851
(2009). http://dx.doi.org/10.1021/la9019475 LANGD5 0743-7463 Google Scholar
R. Jin,
“Quantum sized, thiolate-protected gold nanoclusters,”
Nanoscale, 2
(3), 343
–362
(2010). http://dx.doi.org/10.1039/b9nr00160c 1556-276X Google Scholar
J. Akolaet al.,
“On the structure of thiolate-protected ,”
J. Am. Chem. Soc., 130
(12), 3756
–3757
(2008). http://dx.doi.org/10.1021/ja800594p JACSAT 0002-7863 Google Scholar
J. F. ParkerC. A. Fields-ZinnaR. W. Murray,
“The story of a monodisperse gold nanoparticle: ,”
Accounts. Chem. Res., 43
(9), 1289
–1296
(2010). http://dx.doi.org/10.1021/ar100048c ACHRE4 0001-4842 Google Scholar
M. Zhuet al.,
“Conversion of anionic cluster to charge neutral cluster via air oxidation,”
J. Phys. Chem. C, 112
(37), 14221
–14224
(2008). http://dx.doi.org/10.1021/jp805786p 1932-7447 Google Scholar
P. D. Jadzinskyet al.,
“Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution,”
Science, 318
(5849), 430
–433
(2007). http://dx.doi.org/10.1126/science.1148624 SCIEAS 0036-8075 Google Scholar
A. A. Herzinget al.,
“Identification of active gold nanoclusters on iron oxide supports for CO oxidation,”
Science, 321
(5894), 1331
–1335
(2008). http://dx.doi.org/10.1126/science.1159639 SCIEAS 0036-8075 Google Scholar
M. Turneret al.,
“Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters,”
Nature, 454
(7207), 981
–984
(2008). http://dx.doi.org/10.1038/nature07194 NATUAS 0028-0836 Google Scholar
Y. ZhuH. QianR. Jin,
“Catalysis opportunities of atomically precise gold nanoclusters,”
J. Mater. Chem., 21
(19), 6793
–6799
(2011). http://dx.doi.org/10.1039/c1jm10082c JMACEP 0959-9428 Google Scholar
D.-en Jianget al.,
“The smallest thiolated gold superatom complexes,”
J. Phys. Chem. C, 113
(40), 17291
–17295
(2009). http://dx.doi.org/10.1021/jp9035937 1932-7447 Google Scholar
G. A. SimmsJ. D. PadmosP. Zhang,
“Structural and electronic properties of protein/thiolate-protected gold nanocluster with ‘staple’ motif: A XAS, L-DOS, and XPS study,”
J. Chem. Phys., 131
(21), 214703
(2009). http://dx.doi.org/10.1063/1.3268782 JCPSA6 0021-9606 Google Scholar
A. Dass,
“Mass spectrometric identification of molecular gold nanoclusters with 34-electron shell closing,”
J. Am. Chem. Soc., 131
(33), 11666
–11667
(2009). http://dx.doi.org/10.1021/ja904713f JACSAT 0002-7863 Google Scholar
M. A. MacDonaldet al.,
“The structure and bonding of nanoclusters from EXAFS: the interplay of metallic and molecular behavior,”
J. Phys. Chem. C, 115
(31), 15282
–15287
(2011). http://dx.doi.org/10.1021/jp204922m 1932-7447 Google Scholar
Z. WuR. Jin,
“On the ligand’s role in the fluorescence of gold nanoclusters,”
Nano Lett., 10
(7), 2568
–2573
(2010). http://dx.doi.org/10.1021/nl101225f NALEFD 1530-6984 Google Scholar
E. S. Shibuet al.,
“Ligand exchange of leading to functionalized gold clusters: spectroscopy, kinetics, and luminescence,”
J. Phys. Chem., 112
(32), 12168
–12176
(2008). http://dx.doi.org/10.1021/jp8045033 JPCHAX 0022-3654 Google Scholar
L. ShangS. DongG. U. Nienhaus,
“Ultra-small fluorescent metal nanoclusters: synthesis and biological applications,”
Nano Today, 6
(4), 401
–418
(2011). http://dx.doi.org/10.1016/j.nantod.2011.06.004 1748-0132 Google Scholar
E. KatzI. Willner,
“Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications,”
Angew. Chem. Int. Ed., 43
(45), 6042
–6108
(2004). http://dx.doi.org/10.1002/(ISSN)1521-3773 ACIEF5 1433-7851 Google Scholar
M. B. DickersonK. H. SandhageR. R. Naik,
“Protein- and peptide-directed syntheses of inorganic materials,”
Chem. Rev., 108
(11), 4935
–4978
(2008). http://dx.doi.org/10.1021/cr8002328 CHREAY 0009-2665 Google Scholar
X. Gaoet al.,
“In vivo cancer targeting and imaging with semiconductor quantum dots,”
Nat. Biotechnol., 22
(8), 969
–976
(2004). http://dx.doi.org/10.1038/nbt994 NABIF9 1087-0156 Google Scholar
Y.-W. LinC.-C. HuangH.-T. Chang,
“Gold nanoparticle probes for the detection of mercury, lead and copper ions,”
Analyst, 136
(5), 863
–871
(2011). http://dx.doi.org/10.1039/c0an00652a ANLYAG 0365-4885 Google Scholar
F. Wanget al.,
“Luminescent nanomaterials for biological labelling,”
Nanotechnology, 17
(1), R1
–R13
(2006). http://dx.doi.org/10.1088/0957-4484/17/1/R01 NNOTER 0957-4484 Google Scholar
N. GoswamiR. SahaS. K. Pal,
“Protein-assisted synthesis route of metal nanoparticles: exploration of key chemistry of the biomolecule,”
J. Nanopart. Res., 13
(10), 5485
–5495
(2011). http://dx.doi.org/10.1007/s11051-011-0536-3 JNARFA 1388-0764 Google Scholar
H. Weiet al.,
“Time-dependent, protein-directed growth of gold nanoparticles within a single crystal of lysozyme,”
Nat. Nanotechnol., 6
(2), 93
–97
(2011). http://dx.doi.org/10.1038/nnano.2010.280 1748-3387 Google Scholar
K. ChaudhariP. L. XavierT. Pradeep,
“Understanding the evolution of luminescent gold quantum clusters in protein templates,”
ACS Nano., 5
(11), 8816
–8827
(2011). http://dx.doi.org/10.1021/nn202901a 1936-0851 Google Scholar
F. Bensebaaet al.,
“XPS study of metal-sulfur bonds in metal-alkanethiolate materials,”
Surface Sci., 405
(1), L472
–L476
(1998). http://dx.doi.org/10.1016/S0039-6028(98)00097-1 SUSCAS 0039-6028 Google Scholar
K. M. HarknessD. E. CliffelJ. A. McLean,
“Characterization of thiolate-protected gold nanoparticles by mass spectrometry,”
Analyst, 135
(5), 868
–874
(2010). http://dx.doi.org/10.1039/b922291j ANLYAG 0365-4885 Google Scholar
Y.-T. Taoet al.,
“Structure evolution of aromatic-derivatized thiol monolayers on evaporated gold,”
Langmuir, 13
(15), 4018
–4023
(1997). http://dx.doi.org/10.1021/la9700984 LANGD5 0743-7463 Google Scholar
T. Yanget al.,
“Synthesis, characterization, and self-assembly of protein lysozyme monolayer-stabilized gold nanoparticles,”
Langmuir, 23
(21), 10533
–10538
(2007). http://dx.doi.org/10.1021/la701649z LANGD5 0743-7463 Google Scholar
J. L. Burtet al.,
“Noble-metal nanoparticles directly conjugated to globular proteins,”
Langmuir, 20
(26), 11778
–11783
(2004). http://dx.doi.org/10.1021/la048287r LANGD5 0743-7463 Google Scholar
A. G. Tkachenkoet al.,
“Multifunctional gold nanoparticle-peptide complexes for nuclear targeting,”
J. Am. Chem. Soc., 125
(16), 4700
–4701
(2003). http://dx.doi.org/10.1021/ja0296935 JACSAT 0002-7863 Google Scholar
L. Shanget al.,
“pH-dependent protein conformational changes in albumin: gold nanoparticle bioconjugates: a spectroscopic study,”
Langmuir, 23
(5), 2714
–2721
(2007). http://dx.doi.org/10.1021/la062064e LANGD5 0743-7463 Google Scholar
N. WangooC. R. SuriG. Shekhawat,
“Interaction of gold nanoparticles with protein: a spectroscopic study to monitor protein conformational changes,”
Appl. Phys. Lett., 92
(13), 133104
(2008). http://dx.doi.org/10.1063/1.2902302 APPLAB 0003-6951 Google Scholar
J. H. Fendler,
“Biomineralization inspired preparation of nanoparticles and nanoparticulate films,”
Curr. Opin. Solid. St. M., 2
(3), 365
–369
(1997). http://dx.doi.org/10.1016/S1359-0286(97)80129-5 COSSFX 1359-0286 Google Scholar
D. BhattacharyaR. K. Gupta,
“Nanotechnology and potential of microorganisms,”
Crit. Rev. Biotechnol., 25
(4), 199
–204
(2005). http://dx.doi.org/10.1080/07388550500361994 CRBTE5 0738-8551 Google Scholar
W. J. Crookes-GoodsonJ. M. SlocikR. R. Naik,
“Bio-directed synthesis and assembly of nanomaterials,”
Chem. Soc. Rev., 37
(11), 2403
–2412
(2008). http://dx.doi.org/10.1039/b702825n CSRVBR 0306-0012 Google Scholar
C.-L. ChenN. L. Rosi,
“Peptide-based methods for the preparation of nanostructured inorganic materials,”
Angew. Chem. Int. Ed., 49
(11), 1924
–1942
(2010). http://dx.doi.org/10.1002/anie.200903572 ACIEF5 1433-7851 Google Scholar
M. A. AlbrechtC. W. EvansC. L. Raston,
“Green chemistry and the health implications of nanoparticles,”
Green Chem., 8
(5), 417
–432
(2006). http://dx.doi.org/10.1039/b517131h GRCHFJ 1463-9262 Google Scholar
J. XieY. ZhengJ. Y. Ying,
“Protein-directed synthesis of highly fluorescent gold nanoclusters,”
J. Am. Chem. Soc., 131
(3), 888
–889
(2009). http://dx.doi.org/10.1021/ja806804u JACSAT 0002-7863 Google Scholar
D. Huet al.,
“Highly selective fluorescent sensors for based on bovine serum albumin-capped gold nanoclusters,”
Analyst, 135
(6), 1411
–1416
(2010). http://dx.doi.org/10.1039/c000589d ANLYAG 0365-4885 Google Scholar
A. Retnakumariet al.,
“Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging,”
Nanotechnology, 21
(5), 055103
(2010). http://dx.doi.org/10.1088/0957-4484/21/5/055103 NNOTER 0957-4484 Google Scholar
M. A. H. Muhammedet al.,
“Luminescent quantum clusters of gold in bulk by albumin-induced core etching of nanoparticles: metal ion sensing, metal-enhanced luminescence, and biolabeling,”
Chem. Eur. J., 16
(33), 10103
–10112
(2010). http://dx.doi.org/10.1002/chem.201000841 CEUJED 0947-6539 Google Scholar
X. Wanget al.,
“Ultrasensitive fluorescence detection of glutaraldehyde in water samples with bovine serum albumin-Au nanoclusters,”
Microchem. J., 99
(2), 327
–331
(2011). http://dx.doi.org/10.1016/j.microc.2011.06.004 MICJAN 0026-265X Google Scholar
X. Le Guévelet al.,
“Formation of fluorescent metal nanoclusters capped in bovine serum albumin followed by fluorescence and spectroscopy,”
J. Phys. Chem. C, 115
(22), 10955
–10963
(2011). http://dx.doi.org/10.1021/jp111820b 1932-7447 Google Scholar
H. Weiet al.,
“Lysozyme-stabilized gold fluorescent cluster: synthesis and application as sensor,”
Analyst, 135
(6), 1406
–1410
(2010). http://dx.doi.org/10.1039/c0an00046a ANLYAG 0365-4885 Google Scholar
C. Shaoet al.,
“Eggshell membrane as a multimodal solid state platform for generating fluorescent metal nanoclusters,”
J. Mater. Chem., 21
(9), 2863
–2866
(2011). http://dx.doi.org/10.1039/c0jm04071a JMACEP 0959-9428 Google Scholar
X. L. GuévelN. DaumM. Schneider,
“Synthesis and characterization of human transferrin-stabilized gold nanoclusters,”
Nanotechnology, 22
(27), 275103
(2011). http://dx.doi.org/10.1088/0957-4484/22/27/275103 NNOTER 0957-4484 Google Scholar
P. L. Xavieret al.,
“Luminescent quantum clusters of gold in transferrin family protein, lactoferrin exhibiting FRET,”
Nanoscale, 2
(12), 2769
–2776
(2010). http://dx.doi.org/10.1039/c0nr00377h 1556-276X Google Scholar
H. Kawasakiet al.,
“Trypsin-stabilized fluorescent gold nanocluster for sensitive and selective detection,”
Anal. Sci., 27
(6), 591
–596
(2011). http://dx.doi.org/10.2116/analsci.27.591 ANSCEN 0910-6340 Google Scholar
H. Kawasakiet al.,
“pH-dependent synthesis of pepsin-mediated gold nanoclusters with blue green and red fluorescent emission,”
Adv. Funct. Mater, 21
(18), 3508
–3515
(2011). http://dx.doi.org/10.1002/adfm.201100886 AFMDC6 1616-3028 Google Scholar
C.-L. Liuet al.,
“Insulin-directed synthesis of fluorescent gold nanoclusters: preservation of insulin bioactivity and versatility in cell imaging,”
Angew. Chem. Int. Ed., 50
(31), 7056
–7060
(2011). http://dx.doi.org/10.1002/anie.v50.31 ACIEF5 1433-7851 Google Scholar
F. Wenet al.,
“Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing,”
Anal. Chem., 83
(4), 1193
–1196
(2011). http://dx.doi.org/10.1021/ac1031447 ANCHAM 0003-2700 Google Scholar
Z. Zhonget al.,
“The surface chemistry of Au colloids and their interactions with functional amino acids,”
J. Phys. Chem. B, 108
(13), 4046
–4052
(2004). http://dx.doi.org/10.1021/jp037056a JPCBFK 1520-6106 Google Scholar
R. L. Willettet al.,
“Differential adhesion of amino acids to inorganic surfaces,”
PNAS, 102
(22), 7817
–7822
(2005). http://dx.doi.org/10.1073/pnas.0408565102 Google Scholar
R. R. Naiket al.,
“Peptide templates for nanoparticle synthesis derived from polymerase chain reaction-driven phage display,”
Adv. Funct. Mater., 14
(1), 25
–30
(2004). http://dx.doi.org/10.1002/(ISSN)1616-3028 AFMDC6 1616-3028 Google Scholar
S. BrownM. SarikayaE. Johnson,
“A genetic analysis of crystal growth,”
J. Mol. Biol., 299
(3), 725
–735
(2000). http://dx.doi.org/10.1006/jmbi.2000.3682 JMOBAK 0022-2836 Google Scholar
Y. N. TanJ. Y. LeeD. I. C. Wang,
“Uncovering the design rules for peptide synthesis of metal nanoparticles,”
J. Am. Chem. Soc., 132
(16), 5677
–5686
(2010). http://dx.doi.org/10.1021/ja907454f JACSAT 0002-7863 Google Scholar
N. K. Chakiet al.,
“Ubiquitous 8 and 29 kDa gold: alkanethiolate cluster compounds: mass-spectrometric determination of molecular formulas and structural implications,”
J. Am. Chem. Soc., 130
(27), 8608
–8610
(2008). http://dx.doi.org/10.1021/ja8005379 JACSAT 0002-7863 Google Scholar
J. W. Hudgenset al.,
“Reaction mechanism governing formation of 1,3-bis(diphenylphosphino)propane-protected gold nanoclusters,”
Inorg. Chem., 50
(20), 10178
–10189
(2011). http://dx.doi.org/10.1021/ic2018506 INOCAJ 0020-1669 Google Scholar
Y. NegishiK. NobusadaT. Tsukuda,
“Glutathione-protected gold clusters revisited: bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals,”
J. Am. Chem. Soc., 127
(14), 5261
–5270
(2005). http://dx.doi.org/10.1021/ja042218h JACSAT 0002-7863 Google Scholar
C. Sunet al.,
“Controlling assembly of paired gold clusters within apoferritin nanoreactor for in vivo kidney targeting and biomedical imaging,”
J. Am. Chem. Soc., 133
(22), 8617
–8624
(2011). http://dx.doi.org/10.1021/ja200746p JACSAT 0002-7863 Google Scholar
A. M. P. Hussainet al.,
“Au-nanocluster emission based glucose sensing,”
Biosens. Bioelectron., 29
(1), 60
–65
(2011). http://dx.doi.org/10.1016/j.bios.2011.07.066 BBIOE4 0956-5663 Google Scholar
I. Sakanagaet al.,
“Photoluminescence from excited energy bands in Nanoclusters,”
Appl. Phys. Express, 4
(9), 095001
(2011). http://dx.doi.org/10.1143/APEX.4.095001 1882-0778 Google Scholar
S. Heet al.,
“Design of a gold nanoprobe for rapid and portable mercury detection with the naked eye,”
Chem. Commun.,
(40), 4885
–4887
(2008). http://dx.doi.org/10.1039/B811528A Google Scholar
C.-C. HuangH.-T. Chang,
“Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles,”
Chem. Commun.,
(12), 1215
–1217
(2007). http://dx.doi.org/10.1039/B615383F Google Scholar
J.-S. LeeM. S. HanC. A. Mirkin,
“Colorimetric detection of mercuric ion () in aqueous media using DNA-functionalized gold nanoparticles,”
Angew. Chem. Int. Ed., 46
(22), 4093
–4096
(2007). http://dx.doi.org/10.1002/(ISSN)1521-3773 ACIEF5 1433-7851 Google Scholar
D. LiA. WieckowskaI. Willner,
“Optical analysis of ions by oligonucleotide-gold-nanoparticle hybrids and DNA-based machines,”
Angew. Chem. Int. Ed., 47
(21), 3927
–3931
(2008). http://dx.doi.org/10.1002/anie.v47:21 ACIEF5 1433-7851 Google Scholar
C.-W. Liuet al.,
“Detection of mercury(II) based on -DNA complexes inducing the aggregation of gold nanoparticles,”
Chem. Commun.,
(19), 2242
–2244
(2008). http://dx.doi.org/10.1039/B719856F Google Scholar
Y. KimR. C. JohnsonJ. T. Hupp,
“Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal ions,”
Nano. Lett., 1
(4), 165
–167
(2001). http://dx.doi.org/10.1021/nl0100116 NALEFD 1530-6984 Google Scholar
J. XieY. ZhengJ. Y. Ying,
“Highly selective and ultrasensitive detection of based on fluorescence quenching of Au nanoclusters by interactions,”
Chem. Commun.,
(46), 961
–963
(2010). http://dx.doi.org/10.1039/B920748A Google Scholar
Y. Liuet al.,
“Gold-nanocluster-based fluorescent sensors for highly sensitive and selective detection of cyanide in water,”
Adv. Funct. Mater., 20
(6), 951
–956
(2010). http://dx.doi.org/10.1002/adfm.200902062 AFMDC6 1616-3028 Google Scholar
M. S. Bakshiet al.,
“Biomineralization of gold nanoparticles by lysozyme and cytochrome C and their applications in protein film formation,”
Langmuir, 26
(16), 13535
–13544
(2010). http://dx.doi.org/10.1021/la101701f LANGD5 0743-7463 Google Scholar
M. Guliet al.,
“Template-directed synthesis of nanoplasmonic arrays by intracrystalline metalization of cross-linked lysozyme crystals,”
Angew. Chem. Int. Ed., 49
(3), 520
–523
(2010). http://dx.doi.org/10.1002/anie.200905070 ACIEF5 1433-7851 Google Scholar
X. TuW. ChenX. Guo,
“Facile one-pot synthesis of near-infrared luminescent gold nanoparticles for sensing copper (II),”
Nanotechnology, 22
(9), 095701
(2011). http://dx.doi.org/10.1088/0957-4484/22/9/095701 NNOTER 0957-4484 Google Scholar
R. Bardhanet al.,
“Fluorescence enhancement by Au nanostructures: nanoshells and nanorods,”
ACS Nano, 3
(3), 744
–752
(2009). http://dx.doi.org/10.1021/nn900001q 1936-0851 Google Scholar
H. DuanS. Nie,
“Etching colloidal gold nanocrystals with hyperbranched and multivalent polymers: a new route to fluorescent and water-soluble atomic clusters,”
J. Am. Chem. Soc., 129
(9), 2412
–2413
(2007). http://dx.doi.org/10.1021/ja067727t JACSAT 0002-7863 Google Scholar
R. Zhouet al.,
“Atomically monodispersed and fluorescent sub-nanometer gold clusters created by biomolecule-assisted etching of nanometer-sized gold particles and rods,”
Chem. Eur. J., 15
(19), 4944
–4951
(2009). http://dx.doi.org/10.1002/chem.v15:19 CEUJED 0947-6539 Google Scholar
X. Yanget al.,
“Blending of and histidine in aqueous solution: a simple approach to the cluster,”
Nanoscale, 3
(6), 2596
–2601
(2011). http://dx.doi.org/10.1039/c1nr10287g 1556-276X Google Scholar
C.-A. J. Linet al.,
“Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications,”
ACS Nano., 3
(2), 395
–401
(2009). Google Scholar
Biography Daniel M. Chevrier is currently a graduate student in the chemistry department at Dalhousie University. He completed a BSc Honours in chemistry at Dalhousie University. While in his undergraduate program, he began his work with Peng Zhang studying iron oxide nanoparticles for their use as drug delivery systems. In 2011, he started his graduate work at Dalhousie University with Peng Zhang and co-supervisor Amares Chatt directing his research towards studying the properties and local structure of fluorescent protein-gold nanoclusters.  Amares Chatt received his BSc (1964, University of Calcutta, India), MSc (1967, University of Roorkee, India), MSc (1970, University of Waterloo, Canada) and PhD (1974, University of Toronto). He has been a full professor (1985 to 2008), Killam Professor of Chemistry (2001 to 2006), director of the SLOWPOKE-2 reactor facility (1987 to 2011), and is currently an adjunct professor of the Department of Chemistry, Dalhousie University in Halifax, Nova Scotia, Canada. His research interests include studies on x-ray spectroscopy of nanomaterials and gamma-ray spectroscopy of radionuclides.  Peng Zhang received his BSc (1993) and MSc (1997) from Jilin University, and PhD (2003) from University of Western Ontario. He was an NSERC postdoctoral fellow at McGill University from 2003 to 2005. Currently, he is an associate professor in the Department of Chemistry, Dalhousie University in Halifax, Nova Scotia, Canada. His research focuses on the x-ray spectroscopy studies of nanomaterials and their applications in catalysis and biomedicine. |